PCR Detection Kit For COVID-19 Coronavirus

Product Details
Urgent Contact
Whatsapp: +8615524105871
+8613042442971
Due to the impact of corona virus and our large order quantity, please contact us via whatsapp to confirm stock and price in real time!
Intended Use
PCR Detection Kit For COVID-19 Coronavirus is suitable for the relative quantitative detection of new coronavirus (SARS-CoV-2) nucleic acid. The test results are for clinical reference only and cannot be used as the basis of diagnosis and treatment alone.
Detection principle
In this kit, fluorescent RT-PCR is used to design specific primers and probe targeting the N gene and ORF1ab gene in the conserved region of the new coronavirus genome. The 5’ end marks FAM (ORF1ab gene) and HEX (N gene) of the probe are detected and compared to the base group, and the 3' end marks are reported to the quenched base group (FAM corresponds to the quenched BHQ1 group; HEX corresponds to the BHQ2 quench group). Before RT-PCR amplification, the fluorescence from 5’ probe emitted by HEX/FAM is absorbed by the quenching group because the quenched group is close to the reporting group, and no fluorescence signal is emitted. When the primer extended, the fluorescence probe bound to the template is cut off by Taq enzyme (5 '→3' exonuclease activity), and the reporter group is separated from the quenched-group to generate fluorescence signal, so as to realize the relative quantitative detection of the novel coronavirus (SARS-CoV-2) at the nucleic acid level in the sample.
Contents of the kit
| Component Name | Size | Number |
| Reaction solution | 650μL | 1 tube |
| Primer probe | 100μL | 1 tube |
| Positive control | 250μL | 1 tube |
| Negative control | 250μL | 1 tube |
Materials required but not provided:Viral RNA extraction kit; RNase and DNase free water; Disposable gloves; RNase and DNase free PCR tube.
Estimated operating time
45minutes to 1 hour
Storage conditions and Expiration
Storage conditions: -15~-30℃, avoid light.
Expiration: 6 months. Avoid repeated freezing-thawing after opening.
Applicable instrument
Bio-Rad CFX96 touch、ABI7500 and Roche et al. fluorescence quantitative PCR instruments with FAM and HEX(VIC) channel.
Sample request
1. Suitable sample types for this kit: upper respiratory tract specimens (including swabs of pharynx, swabs of nose, nasopharynx extracts and deep cough sputum) collected freshly. Lower respiratory tract specimens (including respiratory tract extracts, bronchial lavage fluid, alveolar lavage fluid, lung biopsy specimens) and other samples.
2. After sample collection, the test shall be completed on the same day. Otherwise, it shall be stored in the following condition: 2~8 , no more than 24 hours. Store below -20 for no more than 3 days. Can be stored for a long time below -70 . Repeated freezing-thawing should be avoided.
3. Transportation: the foam box is sealed with ice for transportation.
Operation procedure
1. Sample solution preparation:clinical samples are extracted according to the corresponding requirements and steps with the viral RNA extraction kit, and the extracted RNA could be directly used for detection. If the samples are not immediately detected after extraction, they can also be stored at -70℃, and repeated freezing-thawing should be avoided.
2. Preparation of amplification reagent: take out the reagent from the refrigerator, the reaction liquid and the positive control could be placed on ice to thaw, and the negative control could thaw at room temperature. After thawing thoroughly, mix the mixture upside-down and centrifuge briefly for backup.
3. Sample added: add samples according to the following table, first add negative control, then add samples and positive control. Cover tightly, centrifuge 5 sec at 1800 rpm. After sampling, put the remaining reagent into -20℃ refrigerator for storage immediately .
| Reagent | Samples | Positive control | Negative control |
| —— | 5μL | 5μL | 5μL |
| Reaction solution | 13μL | 13μL | 13μL |
| Primer probe | 2μL | 2μL | 2μL |
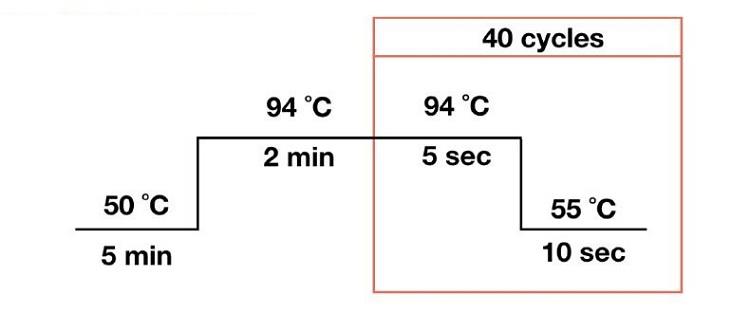
4. RT-PCR amplification (detection part)
Put each reaction tube into the PCR instrument in order, and the reaction is carried out according to the following conditions: when testing with this kit, the FAM channel and HEX channel are both selected for the instrument channel. The reaction volume is 20μL.
Set the parameters as follows:
Quality control
1. Baseline and Threshold adjustment: adjust the Baseline and Threshold according to the image after analysis. The Begin value of the baseline of the bio-rad series of instruments can be 3 and the End value can be 15, and the threshold can be adjusted manually to the point where the threshold line just exceeds the peak of the normal blank control product's FAM-channel /HEX channel amplification curve (irregular noise line).
2. Negative control: neither FAM channel nor HEX channel detected CT value or CT value >40.
3. Positive control: both FAM channel and HEX channel showed typical s-shaped curves with CT value <37.
4. The experiment is valid if 2 and 3 are satisfied at the same time. Otherwise, the experiment is invalid and needs to be repeated.
Interpretation of the results
When the above quality control passed, the following analysis is performed (this kit is for FAM and HEX dual-channel detection) :
1. Positive: if both FAM channel and HEX channel test results of the samples under test are <37, the positive nucleic acid of the novel coronavirus (SARA-CoV-2) can be determined.
2. Negative: if both FAM channel and HEX channel test Ct values are uncountable or Ct values are > 40, and the positive control results are positive, the results will be negative for the new coronavirus (SARS-CoV-2) nucleic acid.
3. Suspicious: if the CT value of the sample to be tested is between 37-40, it is suggested that the sample should be re-extracted and tested again. If the Ct value is <40 and the amplification curve has obvious peaks, the positive nucleic acid of the novel coronavirus (SARS-CoV-2) is determined. Otherwise it's negative.
Limitations of the detection
1. The test results of this product are for clinical reference only and should not be used as the sole basis for clinical diagnosis and treatment. The clinical management of patients should be considered in combination with their symptoms/signs, medical history, other laboratory tests and treatment responses. The test results cannot be directly used as the basis for clinical diagnosis or exclusion of cases, and are only for the reference of clinicians.
2. The test results are related to the collection, storage and transportation conditions of samples, in which any link error can lead to false negative results; False positive results may occur if cross contamination occurs during sample processing.
3. The target sequences detected in this kit are the conserved regions of the novel coronavirus (SARS-CoV-2) N and ORF1ab genes.
Product performance index
1. Detection limit: the detection limit of this kit is 100 copies/mL.
2. Precision: repeatable reference is tested for 10 consecutive times, and its CV of Ct value is less than 10%.
3. The appearance of the kit is good, the liquid components are clear, transparent and non-soluble, and the dosage of each reagent tube is correct.
4. When the kit tested positive control, both the FAM channel and the HEX channel are positive, while when the kit tested negative control, both the FAM channel and the HEX channel are negative.
Caution for handling
1. Before the experiment, please read the kit instructions carefully and strictly follow the operation steps.
2. Specially trained inspectors are required,and the test should be operated in a laboratory with safety protection and protective equipment.
3. The kit should be kept away from light to avoid fluorescence attenuation. The centrifuge tube and tip head used shall be autoclaved and DNase and RNase free. Labelling on the cap of centrifuge tubes should be avoided, which will interfere the fluorescent signal.
4. The samples to be tested in this kit shall be considered as infectious substances, and the operation and treatment shall meet the requirements of the ministry of health's 《General Guidelines for Biosafety of Microbial Biomedical Laboratories》 and 《Regulations on Clinical Waste Management》.
Certificate